What makes a battery work?
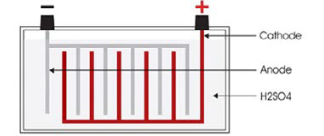
Lead Acid Batteries are basically a chemical storage device. They have three main components that produce a chemical reaction to create electrical current under load:
- Positive (+) Plate/Cathode (Lead Dioxide)
- Negative (-) Plate/Anode (Sponge Lead)
- Electrolyte: Water & Sulphuric Acid (Hydrogen Sulphate) H2SO4
Lead-acid Batteries consist of several single cells connected in series; each cell produces approximately 2.1 volts. Changing the number of plates in each cell alters the battery’s capacity. Cold Cranking Amps (CCA) Ampere Hours (stored energy) and Reserve Capacity (RC) are all increased by adding more plates or larger plates (increasing plate surface area) but the voltage will not change – it will remain at ~2.1V per cell regardless of whether the battery has five tiny plates/cell (small M/Cycle battery) or 39 large plates/cell (large mining truck battery).
The strength or concentration of the sulphuric acid will determine the exact voltage of each cell – weaker diluted acid (1.250SG) will provide approx. 2.10V/cell and stronger 1.300SG acid will provide approx. 2.16V/cell. Stronger acid provides higher voltage and increased cranking power but is also more corrosive, so care must be taken to balance sufficient power with expected battery life when determining the ideal acid SG for a particular application. AGM batteries can tolerate higher SG acid due to the plates being tightly packed between the AGM separators. This holds the plate grids and active material firmly in place for longer, allowing maximum power to be extracted until the battery reaches end of life.
A six-volt battery has three single cells connected in series which produce an output voltage of 6.3 to 6.4 volts when fully charged.
An eight-volt battery has four cells, which produce an output voltage of 8.4 to 8.5 volts (fully charged).
A twelve-volt battery has six cells in series producing a fully charged output voltage of 12.6 to 12.8 volts.
A battery cell contains an element consisting of positive and negative lead plates, with separators in between to prevent the plates from touching and shorting out. The elements are enclosed in a plastic battery case and then submersed in an electrolyte. The more plates in each element the more cranking and amp-hour capacity it has, but voltage remains the same.
If you would like any further information, please do not hesitate to contact us.